Thomas Laude's pages - Science de la matière et de l'énergie
I. Nanostructures of layered materials
Note : These pages are not an exhaustive review of all the tremendous amount of work that as been published in the field. I welcome any remark, any proposal for links or for bibliography.
Quick jump to I.2 Spherical and tubular morphologies
I.1 Materials known to form closed nanostructures
Terminology: From a strict definition, a "nano-structure" is a particle of nanometer size. This designation has various meanings in literature like, cluster, big molecule, nano-crystal embedded in a matrix... In the specific case of layered materials, nanometre scale structures are topologically closed and hollow. They typically form tubular or spherical morphologies. It is especially the case for carbon, which presents a remarkable diversity of structures on the nanometre scale.
I.1.1 Graphite and hexagonal boron nitride (h-BN)
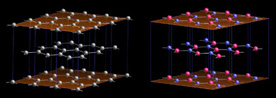 |
| Similarities between h-BN and graphite The nearest neighbour distances (0.144 and 0.142 nm respectively) and the inter-layer spacings (c/2=0.33 and 0.335 nm) are almost identical. |
BN is a structural equivalent of carbon. It is mostly found in the same phases, and produces similar nano-structures. This is probably because, the B-N bounding tends to be dipolar, and averages the number of electron per atom to 2 in the 2P layer, as for carbon. (carbon is 2P2, nitrogen is 2P3, bore is 2P1)
At low temperature and pressure, h-BN is a stable phase, although solid boron + nitrogen is clearly meta-stable. (A piece of boron under ambient air does not recombine.) The hexagonal phase of BN is very similar to graphite. (It is sometime called "white graphite", for its aspect.) Crystallographic parameters are almost equal (~ 1%). However, in h-BN, hexagons of neighbouring planes are superposed (B and N atoms in succession along the c axis), when in graphite they are shifted of half a hexagon.
High-pressure phases of both materials have been intensively studied for their interest as ultra hard material. Both materials are stable in a dense cubic phase (c-BN and diamond), although BN also forms a metastable hexagonal phase, known as wurtzite-type.
At high temperatures but low pressures (case of the present study), carbon and BN phases differ. BN tends to dissociate, when carbon is stable as a gas. Even at temperatures where both elements are vaporised, a BN molecule is unstable (JANAF, for 1 bar [1.1.3]). A phase diagram, for high temperature and low pressure, can be deduced from vapour pressure measurements of B, BN and C. [1.1.4] Vapour pressure measurements give the equilibrium conditions of a vapour/liquid (or vapour/solid) interface, all phases being at uniform temperature. In the present case, when rising temperature over dissociation temperature of BN (2700 K at 100 mbar of nitrogen), elements are segregated as liquid boron and nitrogen gas. When getting over evaporation temperature of boron (3500 K at 100 mbar of B gas), boron and nitrogen are independent gases.
At high temperatures and high pressures, both carbon and BN are both reported as a "liquid" phase. Isotropic carbon phase is known to be dominated by molecules, mainly C2. Similarly, as liquid BN phase may be dominated by a stable BN molecule. The transition between segregated and liquid phase of BN, when rising pressure, is not known.
I.1.2 Merits of BN
BN is intensively used as a carbon substitute for its much higher chemical inertness, especially at high temperature. h-BN is non-reactive to molten metals (Al, Fe, Cu, Zn), to hot Si and stable against air oxidation up to 1000° C. (For comparison, graphite burns from 500°C, MoS2 from 350°C and WS2 from 420°C.) BN is typically an interesting material for high temperature applications.(See [1.1.5] and [1.1.6])
For nano-structures, oxidation actually starts at lower temperatures than for bulk material, because of bounding weaknesses at strong particle curvatures. Carbon tubes burn for 400°C [6.7.4], and WS2 onions burn at 320°C [2.2].
h-BN also offers an electrically resistant counterpart to the semi-metallic graphite. This difference is more or less preserved for nano-tubes. BN SWNT are insulators, with a large band gap (~ 5.5 eV). Its resistive character is not much affected by the structural specificity of the tube (helicity, defects, multi-layering・. [4.12.2] By contrast, carbon SWNT are semi-conductors or conductors depending on their helicity and diameter. Multi-layering and rope assembling tend to increase conductivity. [6.10] However, practically, it is not yet possible to synthesis selectively SWNT with specific structure.
I.1.3 B/C/N tubes [5] and other layered materials: MX2 and MCl2 [7]
Substitution of BN in carbon nano-tube has been studied by a number of authors. Tubes with various concentrations of elements have been obtained. It seems that C and BN rather tend to segregate as different hexagonal planes, rather than being uniformly distributed in one plane.
The metal dichalcogenids family, MX2 (M is a metal: Mo or W. X is a dichalcogen: S, Se or Te.), and the metal chloride family, MCl2, are also layered material with a hexagonal arrangement intra-layer. Some onions and nano-tubes tubes have been found in tungsten disulphide and molybdenum disulphide (WS2 and MoS2). Such tubes were short and thick. Similar onions have been found in NiCl2.
I.2 Spherical and tubular morphologies
On nanometre size, structures are formed of concentric hexagonal layers, folded as tubes or as spheres. They are not encountered as a few structures of real repeatability, but rather as wide range of structures. Therefore, classifications are subjective and denominations partly differ from author to author.
I.2.1 Tubes

|
| Click on this image for Steffen Weber's SWNT gallery |
Tubular forms of carbon, commonly called "Nano-tubes", are known since the development of high resolution TEM. For instance, they were already described by A. Oberlin and M. Endo. [6.4.1], as early as 1976. (They also proposed a growth model still relevant today.)
Tubes are cylinders of concentric hexagonal layers (one to several tens of layers), with diameter in the order of the nanometre. Tube lengths are macroscopic, so that the aspect ratio (length / diameter), can be up to 105. For energy considerations, tubes are believed to be several concentric layers, rather than of one spiralling layer. (Because of the cost of the layer edge inside and outside the tube.) This is commonly admitted, although it can not be clearly confirmed by TEM imaging. (It would require observing many section views.)
Boundary limits after one rotation around the axis constraint a limited number of choices for the helicity of the hexagonal layer relatively to tube axis. Indeed, there must be a continuity of hexagons on the cylinder. This limits the number of possible choices for helicity. Any vector joining two equivalent atoms in a graphene plane can form the circumference of a tube. (Of course, this only has a physical meaning if the diameter of the tube is neither too small nor too big.) Then, the axis of the tube is defined perpendicular to this vector. Each vector in the graphene plane defines one helicity of the tube around its axis. There are two specific cases of helicity: when the vector is perpendicular to the border of one hexagon ("Armchair" type) and when the vector crosses to atoms opposed in the hexagon ("Zigzag" type).
In a multi-walled nano-tube (MWNT, by opposition to single-walled nano-tubes, SWNT), it is usually observed that layer inter-distance respects the c interlayer distance of graphite. A disordered layer piling ("turbostratic" graphite) would cause a slightly wider interlayer distance. This means that a tube has to find some accommodations to respect the relative position of two neighbouring layers. For most cases, the misfit in circumference between two neighbouring layers does not correspond to an additional row of hexagon on the upper roll. Compensation may be possible through defects, or through a change of helicity between layers. (See [6.16.3] for accommodation considerations.) The inter-layer accommodation of BN may be different from carbon, because hexagons are superposed and not shifted of half a hexagon.
I.2.2 Onions (typically 10 to 300 nm)
"Onions" are particles roughly rounded, constituted of atomic layers piled as an onion. Morphologies are various. Often, an onion is closed by facets and angles, rather than by a continuous curvature, forming a "nano-polyhedron". This is especially true for diatomic materials like BN, with no "pentagon flexibility": Pentagons allow a hexagonal plane to curve. But for BN, the formation of a pentagon is thought to be energetically costly (because of a B-B or N-N bounding).
Onions are often irregular and full of defects. This is because such structure difficulty accommodates the interlayer constraints in case of spherical curvatures. (See [6.16.3] for accommodation considerations.) Onions probably feature disordered piling of layers (turbostratic). Highly disordered onions are known to exist in common carbon soot. These are very large (several microns), but share the same topology, being spherical and hollow.
I.2.3 The fullerene family [8]
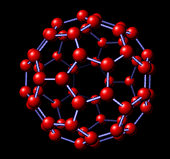
|
| Click on this image for Steffen Weber's fullerene gallery |
The carbon fullerene family is a group of molecule, with chemical formula C2n, (20 < n < 50). Amongst those, a C60 molecule, shaped as a "football ball", 1 nm in diameter (dense sphere model), is the most symmetrical and the most stable structure. It is the one obtained in highest yield for most synthesis methods (usually followed by the C70, shaped as a "rugby balloon").
For BN, pentagons are thought to be energetically costly. However, square and octagon may be viable in a rounded structure, so that some BN closed molecules ~ 1 nm large, have been proposed to exist. [4.11.1]. Those structures are described with sharp angles and low symmetry. However, for such a dimension, the experimental characterisation by TEM imaging of an individual structure is not affordable. (No lattice of such particles was observed.)
I.2.4 C60 and carbon SWNT lattices

|
| Click on this image for Rice University Gallery |
C60 can assemble in a crystalline lattice. It is simple cubic at low temperature, and becomes FCC over 250 K. This is because, at high temperatures, molecules are free spinning, and become isotropic spheres to each other.
Similarly carbon tubes have a natural tendency to self assemble cylinder against cylinder, as a "rope" (also called "bundle"). Carbon SWNT with uniform diameters, form a non-directional 2D triangular lattice in the section of a rope. (See for instance [6.2.8], [6.3.1] and [6.8.3])
To contact me: thomaslaudeABC@uminokai.net (Remove ABC, it is against spam.)
The material present here is copyrighted. Key-words: nanotechnology, science, nanotubes.
Birth of the site: August 1999!
Cours de maths-physique-chimie à Rodez |
Cours de flute à Rodez |
Location Honfleur |
Zameho Folk |
Site Scientifique |
Japonais à Rodez |
lire Avec les amis |
Zameho Folk facebook |
Orchestre country Sailor Step |
Thomas Zameho Facebook |
Production Zameho |
Zameho livetonight |
Zameho Amazon |
Spectable |
acteur-fete |
Musique-XYZ |
country-france |
Zameho r-camping |
livetonight Sailor Step
