Thomas Laude's pages - Science de la matière et de l'énergie
T. Laude 05 November 2003
Boron Nitride (BN) Versus Carbon NT:
Review, and Development Perspectives
Introduction
Boron nitride is a structural equivalent of carbon. It forms similar nano-morphologies: nanotubes, onions, ropes,… BN presents interesting characteristics for applications. In particular, BN is more chemically inert than carbon. For instance, it is stable against oxidation (up to 900C, when graphite burns at 600°C), which makes it a good candidate for high temperature applications. Also, BN presents higher polarity than carbon, possibly allowing better solubility. Although research on BN NT started parallely to research on carbon NT, the number of publications has kept lower of an order. On one hand, BN requires more energy in pre-development research, in particular for mass production, but on the other hand, patenting is more opened.
1. BN, Macro Structures
B
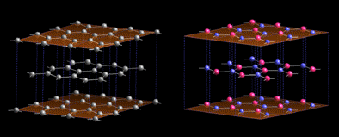
Fig.
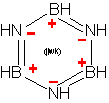
1 Similarities between h-BN (right) and graphite (left) The
nearest neighbour distances (0.144 and 0.142 nm respectively) and
the inter-layer spacings (c/2 = 0.33 and 0.335 nm) are almost
identical. However, in h-BN hexagons of neighbouring planes are
superposed (boron and nitrogen alternated along c axis), when they
are shifted of half a hexagon in graphite.
N is mostly found in the same phases as
carbon, and produces similar structures. This is because the B-N
bounding tends to be dipolar, and averages the number of electron per
atom to 2 in the 2P layer, as for carbon. (carbon is 2P2,
nitrogen is 2P3,
boron is 2P1)

1.1 At low temperature and pressure
h-BN (Fig. 1) is a stable phase, although solid boron + nitrogen is clearly meta-stable. (A piece of boron under ambient air does not recombine.) The hexagonal phase of BN is very similar to graphite. (It is sometime called "white graphite", for its aspect.) Crystallographic parameters are almost equal (~ 1%). However, in h-BN, hexagons of neighbouring planes are superposed (B and N atoms in succession along c axis), when in graphite they are shifted of half a hexagon.
1.2 At high pressure
H
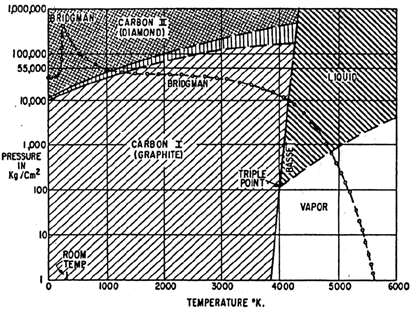
Fig.
3 Phases of carbon according to [1.1.2]
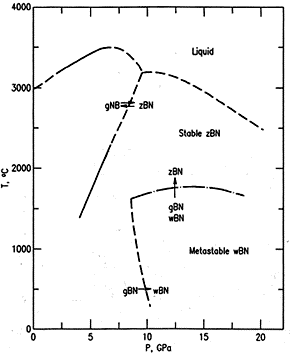
Fig.
2 BN phases at high pressure according to [1.1.1]
(unusual notations: gBN is hexagonal type, wBN is hexagonal wurtzite
type, zBN is cubic type)
1.3 At high temperature and low pressure
At high temperatures but low pressures (case of the NT growth), carbon and BN phases differ. BN tends to dissociate, when carbon is stable as a gas. Even at temperatures where both elements are vaporised, a BN molecule is unstable (JANAF, for 1 bar [1.1.3]). A phase diagram, for high temperature and low pressure, can be deduced from vapour pressure measurements of B, BN and C. (Fig. 4) When rising temperature over dissociation temperature of BN (2700 K at 100 mbar of nitrogen), elements are segregated as liquid boron and nitrogen gas. When getting over evaporation temperature of boron (3500 K at 100 mbar of B gas), boron and nitrogen are independent gases. The recombination of boron with nitrogen requires boron to be liquid.[4.1.2]
1
Fig.
4 Vapours tension measurements for h-BN, B and graphite from
[1.1.4] and most stable form of the B/N system as deduced. [Vapour
pressure measurements give the equilibrium conditions of a
vapour/liquid (or vapour/solid) interface, all phases being at
uniform temperature.]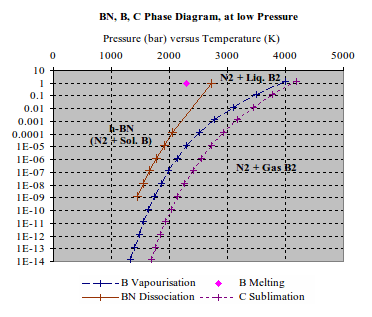
At high temperatures and high pressures (Fig. 2-3), both carbon and BN are reported as a “liquid” phase. Isotropic carbon phase is known to be dominated by molecules, mainly C2. It is not clear if there is liquid BN phase dominated by a stable BN molecule. The transition between segregated and liquid phase of BN, when rising pressure, is not known.
Table 1: Reactions for h-BN production (mostly from [1.1.13])
(Note that N is always in excess)
|
|
Reaction |
Comments |
|
From B |
B + N2 |
T > 2300K (liquid boron) |
|
|
B + NO |
|
|
|
B + NH3 (Ammonia) |
|
|
|
B + N2O |
|
|
|
B + Cyanogens/cyanhydric gas |
750C, poor |
|
From B2O3 |
B2O3 (boron anhydride) + N2 +Coal |
|
|
|
B2O3 + Sodium cyanure |
2000C |
|
|
B2O3 + 3 C + N2 -> 3 CO + 2 BN (or CaB4O7 + 8 C + 3 N2 -> 7 CO + 4 BN + CaCN2) |
1700C |
|
|
B2O3 + 2 NH3 -> 2 BN + 3 H2O |
(Indus.) 900C, purification at 1500C necessary |
|
|
B2O3 + 3CaB6 + 10N2 -> 20BN + 3CaO |
(Indus.) 1500C |
|
|
B2O3 + CaCN2 (Calcium cyamide) (Idem with urete, melamine, oxamide, sodium amidure, sodium nitrade) |
(Indus.) 1200C |
|
From large boranes |
Na2B4O7 (Borax) + 2 NH4Cl (ammonium chloride)->2 NaCl + H2O + 2 NH3 + 2 B2O3; B2O3 + 2 NH3 -> 2 BN + 3 H2O |
(Indus.) Flame heated, 10min, water washing, best purity. May be improved by (tri)calcium phosphate, calcium impurity |
|
|
B2(NH)3, 3HCl (boron imidure chlorhydrate) -> NH4Cl + chlorhydric gas + BN + NH3 |
Thermal decomposition |
|
From BCl3 |
BCl3 (Boron chloride) + NH3 + N2 + H2 |
(CVD) 1000C |
|
|
BCl3 + N2 + H2 |
2000C |
|
|
Boron bromide + NH3 |
(incomplete ref) |
|
|
B3N3H3Cl3 (trichloroborazine) |
Thermal decomposition |
2. BN, Nano-structures
2

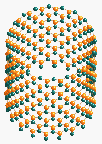

Fig.
5 Three types of BN SWNT Armchair
(10,10), Zigzag (20,0) and a non-specific helicity (15,5).
(Simulation applet by S. Weber)
.1 NT

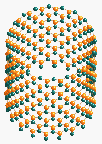

BN NT are most similar to carbon NT. Rolling of layer respects same conditions and hence same helicity rules. (Fig.5)
However, some differences exist:
The existence of SWNT BN is still doubtful. Although two teams reported so [4.2.1 and 4.3.1], proofs are weak. TEM imaging was blurred, inner diameter was 2nm (as for MWNT), neither diffraction or raman was possible (BN is transparent to raman beam). Other teams reported the dominance of double-layered tubes [4.2.6], or of an even number of layer. In thin BN MWNT, arranging of layers by pairs is probable [4.1.3]. This is due to the diatomic character of BN.
Pure stoichiometry is difficult. BN NT often contains boron locally, inside tube or at extremities. [4.2.3]
D
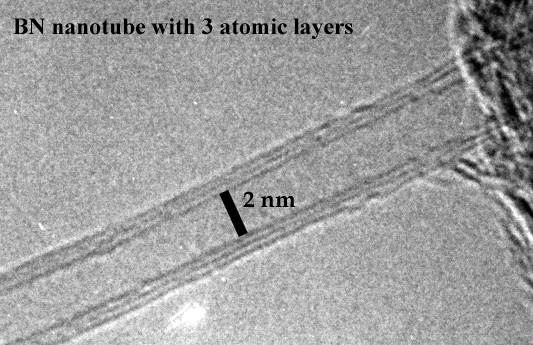
Fig. 6 high resolution TEM image
Fig. 7 TEM imaging of a small BN nano-polyhedron containing a boron nano-crystal Several defects are observable in the shell. It merged on left side with another particle. Scale bar 10 nm. [4.1.2]
ominance of achiral helicity is reported [4.1.3], and in some reports dominance of zigzag in particular.
2.2 Onions (typically 10 to 300 nm)
“Onions” are particles roughly rounded, constituted of atomic layers piled as an onion. Morphologies are various. Often, an onion is closed by facets and angles, rather than by a continuous curvature, forming a “nano-polyhedron”. (Fig. 7) This is especially true for diatomic materials like BN, with no “pentagon flexibility”: Pentagons allow a hexagonal plane to curve. But for BN, the formation of a pentagon is thought to be energetically costly (because of a B-B or N-N bounding). BN onions tend to have a boron core because of non-stoichiometric conditions. [4.1.2]
2.3 Fullerene like BN [8]
The carbon fullerene family is a group of molecule, with chemical formula C2n, (20 < n < 50). Amongst those, a C60 molecule, shaped as a “football ball”, 1 nm in diameter, is the most symmetrical and the most stable structure. For BN, pentagons are thought to be energetically costly. However, square and octagon may be viable in a rounded structure, so that some BN closed molecules ~ 1 nm large, have been proposed to exist. [4.11.1] Those structures are described with sharp angles and low symmetry. For such a dimension, the experimental characterisation by TEM imaging of an individual structure is not affordable. (No lattice of such particles was observed.)
2.4 Synthesis methods (mainly for NT)
For BN, synthesis methods have first been adapted from carbon equivalents, but original methods have also been developed. (BN presents a specific difficulty in that stoichiometry must be achieved between B and N feeders. N is usually fed in excess.) Micron long thin tubes and ropes in macroscopic quantity were obtained mainly by arc discharge method (boron in N2) and by non-ablative laser heating method (h-BN in N2).
2.4.1 Arc discharge methods
The low electric conductivity of h-BN makes difficult using it as an electrode in an arc discharge method. But several alternatives have been found:
Arc discharge on a hollow tungsten electrode filled with h-BN [4.2.1] This was the first method to produce thin BN tubes. Tubes are in the order of 200 nm and have a W particle at their extremity.
Arc discharge on a HfB2 electrode in N2 [4.2.2] This was the first report of BN SWNT. (Doubtful because characterisation was weak.) Tubes are in the order of 700 nm, and mixed with Hf particles. Quantity produced is not known.
Arc discharge on a ZrB2 electrode in N2 [4.2.4] This method is a variant of the previous method, using Zr instead of Hf. Tubes are in the order of 100 nm and mixed with Zr particles.
Arc discharge on B electrode in N2 [4.2.6] This method produces a macroscopic quantity of BN double-layered tubes, several micron long. It also produces BN nano-polyhedrons with a boron core. It is the most developed method at present.
2.4.2 Laser Methods [4.3]
A laser pyrolysis in the gas phase of BCl3 + NH3 (-> BN + 3 HCl) was reported to produces BN onions. (No NT, probably because of no catalyst.) [4.7]
A laser ablation method for BN has been reported, using an excimer (pulsed) laser on h-BN target, under helium or nitrogen. This produces thin tubes merged to the bulk. Tubes (~ 100 nm on photos) are mixed with Ni or Co, depending on use of catalyst. Quantity is not known, but yield is reported high.
Non ablative laser heating (NT grow as a crown around laser impact) [4.1] This method produces extremely long thin tubes thanks to good stability in growing zone. Tubes assemble in ropes. BN nano-polyhedrons with a boron core are also produced.
2.4.3 Furnace and CVD
(1100 C) B2H6+NH3+ZrB2 [4.4.1]. The first method reported for BN tubes. It produces large filaments, quite long (~ 10 m) mixed with ZrB2 particles.
(1200C) B and Li in a BN crucible in N2 atmosphere [4.4.2]. This is not strictly speaking a synthesis method, but conditions in which some BN tubes (~ 30 nm on photos) where observed growing from the bulk. Tubes were found on boron amorphous particles.
(1000C) B previously ball milled in NH3 [4.4.3]. This method produced large BN filaments (~ 5 m).
(1700C) Decomposition of C3N6H6.2H3BO3 (melamine diborate). No catalyst. Strait MWNT with amorphous tip [4.4.4]
(1100C) Borazine (produced in-situ) + nickel boride cat. N2 carrier. MWNT, strait. Low yield. [4.4.5]
(1100-1450C) Reduction of boron oxide by B4C (solide) and CO (gas) + N2. Poor results. [4.4.6]
2.4.4 Other methods
Growth on carbon tube templates in oxidising atmosphere [4.6]. This method grows BN tubes from carbon tubes, through an oxidation process. On carbon SWNT ropes, this transformation is reported to be only partial.
Plasma jet on a h-BN target in inert atmosphere [4.8] Tubes are presents in powders ejected from the target. They are relatively thick (~ 20 nm) and in the order of 1 micron long.
3. Molecular BN
3.1 BN molecule
|
Fig. 8 Borazine |
3.2 Borazine (BHNH)3 and BN polymers
Borazine is a liquid analogue to benzene, but different firstly for its polarity. Thanks to B-N polarity, BN molecules solubility is higher than carbon equivalents. Pratically, borazine is mainly used for the formation of boron nitride films (CVD) and as a monomer in polymerisation.
Borazine can undergo addition and substitution reactions, especially with Cl. Substituted borazines form polymers upon heating in the range 300-400°C. Example of cross -linking scheme: >B-C1+H-N< -> >B-N< . The pyrolysis of borazine and its polymers causes cross linking up to boron nitride. It needs to be carried out in ammonia atmosphere to obtain pure boron nitride.
|
Borazine (BHNH)3 |
|
|
Molecular weight |
80,51 |
|
Boiling point |
55°C |
|
Melting point |
-56°C |
|
Density |
0,86 g/ml at 25°C |
|
Vapor pressure |
210 Torr at 20°C |
|
Flash point |
1,7 °C |
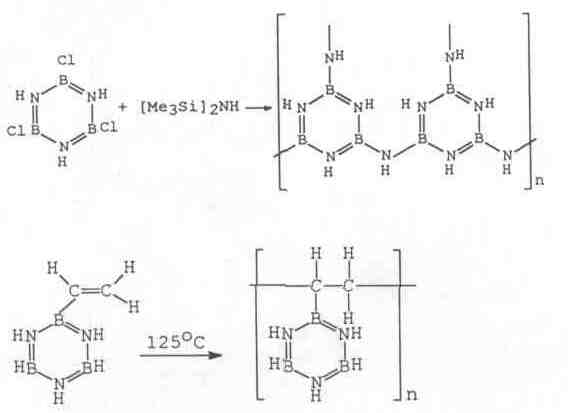
(These macromolecules are soluble in common organic solvents. Polarity also causes more chain rigidity to rotation.)
Borazine is easily available according to the reaction:
T
 3NaBH4
+ 3NH4Cl
B3N3H6
+ 3NaCl + 9H2
3NaBH4
+ 3NH4Cl
B3N3H6
+ 3NaCl + 9H2
Stability: Borazine is a flammable liquid. It is decomposed by water (to form H2, NH3 and boric acid), light, heat, alcohols and acids. Decomposition can occur when stored at ambient temperature for period longer than one month. (Negligible when stored below 0oC.) Borazine is stable in dry air but reacts with trace amount of atmospheric moisture. Slow polymerization may occur by light.
Safety: Liquid borazine may cause burns to skin, eyes and resp. system.
4. BN, properties and merits
4.1. Inertness/Non-wetting
h-BN is already used as a graphite substitute for its higher chemical inertness, especially at high temperature (crucible, …). h-BN is non-reactive to molten metals (Al, Fe, Cu, Zn), molten Si, molten glass, molten salts. h-BN is stable against air oxidation up to 900C. (For comparison, graphite burns from 500°C, MoS2 from 350°C and WS2 from 420°C.) BN is typically an interesting material for high temperature applications. [1.1.5-6] For nano-structures, oxidation actually starts at lower temperatures than for bulk material, because of bounding weaknesses at strong particle curvatures. (Carbon tubes burn for 400°C [6.7.4], and WS2 onions burn at 320°C [2.2].) BN is reactive with fluorine: 2 BN + 3 F2 -> 2BF3 + N2, and fluoric acid BN + 4 HF -> NH4BF4. BN is not reactive with other acids.
4.2 Polarity/Solubility
h-BN is insoluble in standards solvents. (acids, alkalies, benzene, alcohol, acetone,…) However, due to the polarity of the B-N bond, molecular BN is expected more soluble than carbon equivalents. Borazine is readily soluble. Solubility of BN NT has not yet been characterised.
4.3 Resistivity/Dielectricity
h-BN is an electrical resistant counterpart to the semi-metallic graphite. This difference is more or less preserved for nano-tubes. BN SWNT are insulators, with a large band gap (~ 5.5 eV). The resistive character is not affected by the structural specificity of the tube (helicity, defects, multi-layering,…). [4.12.2] By contrast, carbon SWNT are semi-conductors or conductors, depending on helicity and diameter. However, practically, most carbon NT are conductors, because of multi-layering, bundling [6.10], or mixed helicities.
4.4 Non-Toxicity
h-BN is a non-toxic powder sometime used in cosmetics. It may contain several % of boron oxide, which is mild health nuisance. However, boron oxide is washable by boiling water. Also BN NT often contains boron nanoparticles (probably non toxic). As for carbon NT, toxicity is not yet clear. As a volatile fibrous material, carbon NT may cause damage to lung cells, as it is famously the case of asbestos fibres.
4.5 Low price
Carbon is a cheaper element than boron, but both as are found in large quantity on earth. BN is mostly produced from Na2B4O7 (Borax) (See table 1.)
4.6 Transparency and polarisation
Due to the high band gap (5.5 eV), BN (h-BN or NT) only absorb UV and higher energy radiations. It is mostly transparent to visible and IR (absorption on hundreds of microns). As a result, BN NT can not be characterised by raman spectroscopy. On the opposite, the absorption of carbon SWNT depends on the band gap, hence on helicity. If absorbing, both BN and C NT are good polarisers, absorbing light polarised perpendicular to tube axis.
5
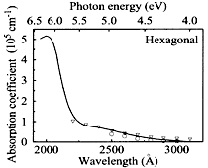
Fig.
9 Absorption of h-BN [Phys. Rev. B 13, 12 (1976), 5560-5573]
. Possible applications for BN NT, a few
examples

Few applications specifically using BN NT have been proposed yet. However, most application proposed for carbon NT are feasible with BN NT. In many of those BN is advantageous. Both carbon and BN meet same practical difficulties. One difficulty is the manipulation of individual structures on the nanometre scale (as required for nano-electronics, for instance). Another difficulty is mass production. A third difficulty is to synthesise selected structures (tube of given length, radius, helicity for instance) or to develop post synthesis techniques for structure segregation.
5.1 Composite materials
As both carbon [6.12] and BN nano-tubes (1.2 TPa [4.10]) have an exceptional elastic modulus, both can be used in mechanical reinforcement. Ceramic composites with h-BN reinforcement are already intensively studied (ex of matrix: ZrO2, SiC, AlN). Typical problems are: mixing in matrix, dispersion of tubes, matrix/tube interface. For these, BN and carbon should differ. Dispersibility and matrix/tube interface may be influenced by the polarity of BN. In addition, the good chemical inertness of BN, may decrease the aging of the composite. On the other hand, BN NT may be more difficult to functionalise.
5.2 Nano-lubricants
Solid lubricants are used when conditions do not allow the usage of standard lubrication oil. This is typically under vacuum or in oxidising atmosphere. h-BN, graphite and WS2 are already intensively used as a solid lubricant in industry. h-BN is especially interesting for having both a very low friction coefficient and a high range of suitable temperature in air (up to 900 °C). [1.1.5] Nano-onions powders are exceptional solid lubricants, because onions act like nanometric ball bearings. This was demonstrated for powders of WS2 nano-onions [2.1][2.2], but it is probably true for the other materials.
5.3 NT Filter
Thanks to their thinnest, NT enable a filtering with an exceptional flux (because the efficient collision surface is very small in the cross section). They could also enable filtering of nano-particles if the network was ideally densified. For instance, such filter could be applied to filter automobile exhausts. BN and C have same mechanical properties. However BN could be advantageous in some cases. For instance, cleaning a BN filter of carbon particles is easily done by temperature oxidation.
5. 4 NT macro fibre, tissue, or fiber mat…
Theoretically, NT may produce any form of fibrous material. Thanks to NT exceptional strength, it may provide exceptional resistance to tearing. It would also offer remarkable resistance to temperature. A NT tissue may be similar to a usual ceramic tissue for many properties. However it may also offer interesting differences, in particular for the softness. Study is needed. As for previous filter application, BN fibres can be cleaned from organics by simple temperature oxidation.
5.5 Hydrogen containers
Industrial age is causing an exponential growth of CO2 concentration in the atmosphere, mainly due to extensive use of fossil energy sources. The “global warming effect” makes research on non-polluting energy sources a priority. Hydrogen is an ideal candidate, because its combustion produces no other release than water. Practically, the main limit to the commercialisation of hydrogen motor is the difficulty for a safe way to store hydrogen. Carbon NT and onions are thought to be a safe storage, because of high H2 trapping capacity. Such storage was measured with variable success, between 0 and 10 wt % in carbon tubes. (See [2.10] for instance.) Such study has not been led with BN NT, although similar H2 trapping properties may be expected.
5.6 Nano-transistors
The conductivity of a carbon SWNT depends on diameter and helicity [6.10.4]. Different carbon NT can theoretically form a nano-sized junction, which is a first step toward "nano-electronic". A nano-transistor was realised through the body of a carbon nano-tube. [2.4] However two major difficulties are expected: manipulation on nanometre scale, and synthesising carbon NT with specific structure. BN is complementary to carbon: It is an insulator of large band gap (~ 5eV, like diamond), little dependent on structure. Diverse possibility of hetero-structures, like C/BN or C/Si are studied. (See [2.6], for instance.) It may also be possible to dope BN NT.
6. Prespectives for BN NT synthesis
(Also see table 1.) To produce stoichiometric BN, N element must be in excess in the gas phase. Hence gas or liq with high vapour pressure is necessary. Typical candidates are N2 and NH3. The presence of metal catalyst may not be necessary. Often, boron itself plays as a catalyst. The main problem is to choose the B source.
6.1 Boron oxide (solid) route
Boron oxide is an interesting B source because:
It is already used as B source in most industrial h-BN production
It was little used for BN NT production (patentable)
It is solvable in boiling water, hence it may play same role as iron/cobalt acetates in ethanol NT production
It melts near 450C, helping formation of boron catalyst
It contains oxide as ethanol in ethanol NT production
It is a safe material
Procedure could be as follow: Mixing of zeolite and boron oxide in boiling water. Drying. (Fitering?) Annealing powder in vacuum near 900C. Flowing NH3.
However, it will not be possible to use HF to remove zeolite from BN NT, because of reactivity.
6.2 The liquid (borazine or BCl3 ) route
Two interesting candidates for a liquid boron source are BCl3 (boil at 13C) and borazine (boil at 55C). (Both have already been used for BN NT production.) We may also use BBr3 (boil at 91C), but it is a health hazard. BCl3 have the advantage to provide only one B atom per molecule (there are also few C atoms in ethanol). As N source, we may use N2 or NH3. Theoretically, it is possible to control the vapor pressure of two species thanks to the double pumping system.
The problem is that the reaction may form in the gas phase, uncatalytically. This would likely forms onions rather than NT. We may use nickel boride as catalyst (as reported in literature) to localise the growth on the catalyst. But the formation of nano nickel boride particle is a technical difficulty to solve.
Additional Notes:
B/C/N tubes [5]
Substitution of BN in carbon nano-tube has been studied by a number of authors. Tubes with various concentrations of elements have been obtained. C and BN tend to segregate as different hexagonal planes, rather than being uniformly distributed in one plane.
Other layered materials: MX2 and MCl2 [7]
The metal dichalcogenids family, MX2 (M is a metal: Mo or W. X is a dichalcogen: S, Se or Te.), and the metal chloride family, MCl2, are also layered material with a hexagonal arrangement intra-layer. Some onions and nano-tubes tubes have been found in tungsten disulphide and molybdenum disulphide (WS2 and MoS2). Such tubes were short and thick. Similar onions have been found in NiCl2.
(Bibliography mostly extracted from T.Laude PhD Thesis)
[1]Datas
[1.1] Physical datas
E. Rapoport, Ann. Chim. (Paris), 10 (7) 607-638 (1985)
Bundy, Hall, Strong and Wentorf (1955)
JANAF thermochenical tables 3tr edition (1985)
J. Margrave, The characterisation of high temperature vapors, p. 502, John Wiley & sons, Inc., New York, London, Sydney
E. A. Smith, Graphite and BN ("white graphite"): aspects of structure, powder size, powder shape, and purity, Powder Metallurgy 14, 27, 110 (1971)
A. Lipp, K. A. Schwetz & K. Hunold, h-BN: fabrication, properties and applications, J. Eur. Ceram. Soc. 5, 3 (1989)
Graphite, Thermophysical Properties of High-Temperature Materials, Vol. 1, 118-121 / 124-131.
Properties of boron nitride, Thermophysical Properties of High-Temperature Materials, Vol. 5, 499-516
Y.S. Touloukian, et al.,Thermophysical properties of matter Vol. 2, IFI/Plenum, NewYork-washington (1970)
Y.S. Touloukian, D.P.Dewitt, Thermophysical properties of matter Vol. 8-Thermal radiative properties Y.S. IFI/Plenum, NewYork-washington (1972)
Handbook of thin film process Technology, A1.5:2, IOP Publishing Ltd (1995)
JCPDS card
P. Pascal, Nouveau traité de chimie minérale VI, Masson, Paris
W. Spitzer and W. Kaiser, Optical properties of crystalline boron, Phys. Rev. Let. 1, 7, 230 (1958) (Errata, 15 nov)
E. Taft, H. Philipp, Optical properties of graphite, Phys. Rev. 138,1A, A197 (1964)
S. Ergun, Optical studies of carbon, Chemistry and physics of carbon Vol.3, Marcel derker, Inc., New York (1968)
K. Oda et al., Oxidation kynetics of h-BN powder, J. of mat. Science, 28, 6562 (1993)
Etude bibliographique des effets d’un flux laser sur une cible, 2ieme partie, Chauffacge, fusion et vaporisation, p. 15, Rapport technique ONERA 5/7184 SY (1980)
Effects of high-power laser irradiation p. 82, J. F. Ready, Academic press New York
Handbook of heat and thermo dynamics 5-14
[1.2] Mathematical references
M. Lax, Temperature rise induced by a laser beam, J. of appl. Phys., 48, 9 3919 (1977), and M. Lax, Temperature rise by a laser beam II. The nonlinear case, Appl. Phys. Let., 33(8), 786 (1978)
H. Ma, S. H. Lin, R. W. Carpenter, P. Rice, O. F. Sandey, Ad. in. calculation of band structure, x-ray emission, quantum yield and EELS of h-BN, J. Appl. Phys. 73, 11, 7422 (1993)
Tarrio, S. E. Schnatterly, Interband transitions, plasmons, and dispersion in h-BN, Phys. Rev B 40, 1, 7852 (1989)
J. C. Slater, G. F. Koster, simplified LCAO method for the periodic potential problem, Phys. Rev. 94, 6, 1498 (1954)
P. R. Wallace, The band theory of graphite, Phys. Rev. 71, 9, 622 (1947)
J. Furthmuller et al., Ab initio calculation of the structural and electronic properties of carbon and boron nitride using ultrasoft pseudopotentials, Phys rev B, 50, 21, 15606 (1994)
[2]Potential for Applications
L. Rapoport, Y. Bilik, Y. Feldman, M. Homyonfer, S.R. Cohen, R. Tenne, Hollow nanoparticles of WS2 as potential solid-state lubricants, Nature 387, 791 (1997) (exist supplementary material on line)
L. Rapoport & al., Inorganic fullerene-like material as additives to lubricants: structure-function relationship, Wear 225-229, 975 (1999)
S. J. Tans et al., Individual single-wall carbon nanotubes as quantum wires, Nature 386, 474 (1997)
S. J. Tans, A. R. M. Verschueren, C. Dekker, Room temperature transistor based on a single carbon NT, Nature 393, 49 (1998)
J. Kong, H. T. Soh, A. M. Cassell, C. F. Quate, H. Dai, Synthesis of individual single-walled carbon nanotubes on patterned silicon wafers, Nature 395, 878 (1998)
Y. Zhang, T. Ichihashi, E. Landree, F. Nihey, S. ijima, Heterostructures of single-walled carbon nanotubes and carbide nanorods, Science 285, 1719 (1999)
H. Dai, J. H. Hafner, A. G. Rinzler, D. T. Colbert, R. E. Smalley, NT as nanoprobes in scanning probe microscopy, Nature 384, 147, (1996)
S. S. Wong, E. Joselevich, A. T. Wooley, C. L. Cheung, C. M. Lieber, Covalently functionalized NT as nanometre-sized probes in chemistry and biology, Nature 394, 52 (1998)
M. Watanabe, M. Tachikawa, T. Osaka, On the possibilty of H intercalation of graphite-like carbon materials(...), Electrrochimica Acta 42, 17, 2707 (1997)
A.C. Dillon, K.M. Jones, T. A. Bekkedahi, C.H. Kiang, D.S. Bethune, M.J. Heben, Storage of hydrogen in single-walled carbon nanotubes, Nature 386, 377 (1997) and Dillon et al. carbon NT materials for hydrogen storage, Proceedings of the 2000 DOE/NREL hydrogen program review NREL/CP-570-28890 (free download at present)
G. Che, B. B. Lakshmi, E. R. Fisher, C. R. Martin, Carbon nanotubule menbranes for electrochemical energy storage and production, Nature 393, 346 (1998)
S. Fan et al., Self-oriented regular arrays of carbon nanotubes and their field emission properties, Science 283, 512 (1999)
B. Rinzler et al., unraveling nanotubes: field emission from an atomic wire, Science, 269, 1550 (1995)
W. Choi et al., Fully sealed, high-brightness carbon-NT field emission display, Appl. Phys. Let., 75, 20, 3129 (1999)
S. Fan et al., Self oriented regular arrays of C NT and their field emission properties, Science 283, 512 (1999)
P. Král and D. tomanek, Laser-driven atomic pump, 82, 26, 5373 (1999)
[4] BN nanostructures
[4.1] synthesis of BN nanotubes by non-ablative laser heating
T. Laude et al., Long ropes of BN NT grown by a continuous laser heating, Appl. Phys. Let. 76, 22, 3239 (2000)
T. Laude, Boron nitride nanotube grown by non-ablative laser heating: Synthesis, Characterisation and Growth processes, PhD Thesis, University of Tsukuba or Ecole Centrale Paris (2001)
T. Laude et al., Fine modulations in the diffraction pattern of boron nitride nanotubes synthesised by non-ablative laser heating, Submitted (2003)
T. Laude et al., Non-uniformity of temperatures along nanotubes in hot reactors and axial growth, Submitted (2003)
[4.2] synthesis of BN nanotubes by arc discharge methods
N. G. Chopra et al., Boron nitride nanotubes, Science 269, 966 (1995)
A. Loiseau, F. Willaime, N. Demoncy, G. Hug, H. Pascard, BN NT with reduced numbers of layers synthesized by arc-discharge , Phys. Rev. Lett. 76, 4737(1996).
A. Loiseau et al., BN NT, Carbon 36, 5-6, 743 (1998)
Y. Saito, M. Maida, T. Matsumoto, Structures of BN NT with single-layer and multi-layers produced by arc discharge, Jpn J. Appl. Phys. 38, 159 (1999)
Y. saito, M. Maida, Square, pentagon, and heptagon rings at BN NT tips, J. of Phys. Chem A 103, 10, 1291 (1999)
J. Cumings, A. Zettl, Mass production of boron nitride double-wall nanotubes and nanococoons, Chem. Phys. Let. 316, 211 (2000)
M. Kuno, et al., Synthesis of boron nitride nanotubes and nanocapsules with LaB6, Diamond and related materials 10 1231-1234 (2001)
[4.3] synthesis of BN nanotubes by laser ablation method
D. P. Yu et al., Synthesis of boron nitride nanotubes by means of excimer laser ablation at high temperature, Appl. Phys. Lett. 72, 16, 1966 (1998)
W. Zhou, Z. Zhang, Z. G. Bai, D. P. Yu, Catalyst effects on formation of BN nano-tubules synthesized by laser ablation, Sol. St. Com. 109, 555 (1999)
[4.4] synthesis of BN nanotubes by oven heating
P. Gleize, M. C. Schouler, P. Gadelle, M. Caillet, Growth of tubular BN filaments, J. of Mat. Science 29, 1575 (1994)
M. Terauchi, M. Tanaka, H. Matsuda, M. Takeda & K. Kimura, Helical nanotubes of hexagonal boron nitride, J. of Elec. Micros. 46, 1, 75 (1997)
Y. Chen, J. F. Gerald, J. S. Williams, S. Bulcock, synthesis of BN NT at low temperatures using reactive ball milling, Chem. Phys. Lett. 299, 260 (1999) and Y. Chen et al., A solid state process for formation of BN NT, Appl. Phys. Let. 74, 20, 2960 (1999)
R. Ma, Y. Bando, T. Sato, CVD synthesis of boron nitride nanotubes without metal catalysts, Chem. Phys. Lett. 337, 61-64 (2001)
O. R Lourie, et al., CVD growth of boron nitride nanotubes, Chem. Mater. 2000, 12, 1808-1810 (2000)
V. Pokropivny, et al. BN analogs of fullerenes (the fulborenes), nanotubes, and fullerites), J. Sol. State Chem. 154, 214-222 (2000)
[4.5] Synthesis of some BN nanotubes by H. P. compression of c-BN micro-crystals in a diamond anvil cell induced by laser heating
D. Golberg et al, Nanotubes in boron nitride laser heated at high pressure, Appl. Phys. Lett. 69, 14, 2045 (1996)
[4.6] synthesis of BN nanotubes by "substitution" of C to BN in oxidizing atmosphere
W. Han, Y. Bando, K. Kurashima, T. Sato, Synthesis of boron nitride nanotubes from carbon nanotubes by a substitution reaction, Appl. Phys. Let.73, 21, 3085 (1998) and D. Goldberg et al., MoO3-promoted synthesis of BN MWNT from C NT templates, Chem. Phys. Let. 323185 (2000)
(See also H. Dai, E. W. Wong, Y. Z. Lu, S. Fan, C. M. Lieber, Synthesis and characterization of carbide nanorods, Nature 375, 769 (1995))
[4.7] synthesis of BN onions by laser pyrolysis in the gas phase
L. Boulanger, B. Andriot, M. Cauchetier, F. Willaime, Concentric shelled and plate-like graphitic BN nanoparticles produced by CO2 laser pyrolysis, Chem. Phys. Let. 234, 227 (1995)
M. I. Baraton et al., Nanometric BN powders: laser synthesis, characterization and FT-IR surface study, J. of the Eur. Ceramic society 371 (1994)
[4.8] synthesis of some BN nanotubes in plasma jet method
Y. Shimizu, Y. Moriyoshi, H. Tanaka, BN NT, webs, and coexisting amorphous phase formed by the plasma jet method, Appl. Phys. Let. 75, 7, 929 (1998)
[4.9] EELS studies
C. Souche, B. Jouffrey, G. Hug, M. Nelhiebel, Orientation sensitive EELS analysis of BN nanometric hollow spheres, Micron 29, 6, 419 (1998)
P. Gleize, S. Herreyre, P. Gadelle, M. Mermoux, Characterization of tubular boron nitride filaments, Mat. Sci. Lett. 13, 1413 (1994)
M. Terauchi, M. Tanaka, T. Matsumoto, Y. Saito, EELS study of the electronique structure of BN NT
[4.10] Mechanical properties
N. G. Chopra & A. Zettl, Measurement of the elastic modulus of a multi-wall BN nanotube, Sol. Sta. Com. 105, 5, 297 (1998)
[4.11] Irradiation experiments
O. Stéphan, Y. Bando, A. Loiseau, F. Willaime, N. Shramchenko, T. Tamiya, T. Sato, Formation of small single-layer and nested BN cages under electron irradiation of nanotubes and bulk material, Appl. Phys. A 67, 107 (1998)
[4.12] Structural calculations
P. W. Fowler, K. M. Rogers, G. Seifert, M. Terrones, H. Terrones, Pentagonal rings and nitrogen excess in fullerene-based BN cages and nanotube caps, Chem. phys. Let. 299, 359 (1999)
A. Rubio, J. L. Corkill, M. Cohen, Theory of graphitic BN NT, Phys. Rev. B 49, 7, 5081 (1994)
[5] B/C/N nanotubes
O. Stéphan, P. M. Ajayan, C. Colliex, P. Redlich, J. M. Lambert, P. Bernier, P. Lefin, Doping graphitic and carbon nanotube structures with boron and nitrogen, Science 266, 1683 (1994)
Z. Weng-Sieh et al., Synthesis of BxCyNz nanotubules, Phys. Rev. B 51, 16, 11229 (1995)
K. Suenaga, C. Colliex, N. Demoncy, A. Loiseau, H. Pascard, F. Willaime, Synthesis of nanoparticles and nanotubes with well-separated layers of BN and carbon, Science 278, 653 (1997)
Golberg, Y. Bando, W. Han, K. Kurashima, T. Sato, Single-walled B-doped carbon, B/N-doped carbon and BN nanotubes synthesized from SW carbon NT through a substitution reaction, Chem. Phys. let. 308, 337 (1999)
W. Han, Y. Bando, K. Kurashima, T. Sato, Boron-doped carbon NT prepared through a substitution reaction, Chem. Phys. Let. 299, 368 (1999)
Y. Miyamoto, A. Rubio, M. L. Cohen, S. G. Louie, Chiral tubules of hexagonal BC2N, Phys. Rev. B 50, 7, 4976 (1994)
Y. Miyamoto, A. rubio, S. G. Louie, M. L. Cohen, Electronic properties of tubule forms of h-BC3, Phys. Rev. B 50, 24, 18360 (1994)
D. Golberg, Y. Bando, K. Kurashima, T. Sasaki, Boron doped carbon fullerenes and nanotubules formed through electron irradiation-induced solid-state phase transformation, Appl. Phys. let. 72, 17, 2108 (1998)
C. Satishkumar et al., Boron-carbon NT from the pyrolysis of C2H2-B2H6 mixtures, Chem. phys. let. 300, 473 (1999)
A.Gindulyte, W. N. Lipscomb, L. Massa, Proposed boron NT, Inorg. Chem. 37, 6544 (1998)
A.Gindulyte, N. Krishnamachari, W. N. Lipscomb, L. Massa, Quantum chemical calculation of proposed multicage boron fullerenes, Inorg. Chem. 37, 6546 (1998)
[7]Other materials
[7.1] MX2
R. Tenne, L. Margulis, M. Genut, G. Hodes, Polyhedral and cylindrical structures of tungsten disulphide, Nature 360, 444 (1992)
L. Margulis, G. Salitra, R. Tenne, M. Talianker, Nested fullerene-like structures, Nature 365, 113 (1993)
[7.2] MCl2
Y. R. Hacohen, E. Grunbaum, R. Tenne, J. Sloan, J. L. Hutchison, Cage structures and nanotubes of NiCl2, Nature 395, 336 (1998)
[7.3] Silicon
S. Osawa, M. Harada, E. Osawa, (SiC)60 an idealized superatom?, Fullerene Sci. & Technol. 3, 2, 225 (1995)
R. Kamalakaran, A. K. Singh, O. N. Srivastava, Formation and characterization of silicon nanoparticles-threads, tubules and possibly silicon fullerene-like structures, J. Phys.: Condens. Matter 7, L529 (1995)
S. Nagase, K. Kobayashi, Si60 and Si60X (X=Ne, F- and Na+), Chem. Phys. Let. 187, 3, 291 (1991)
D. L. Williamson et al., On the nanostructure of pure amorphous silicon, Appl. Phys. Let. 67, 2, 226 (1995)
T. Makimura, Y. Kunii, K. Murakami, Light emission from nanometer-sized silicon particles fabricated by the laser ablation method, Jpn. J. Appl. Phys. 35 (Part 1, No. 9A), 4780 (1996)
K.-M. Ho et al., Structures of medium-sized silicon clusters, Nature 392, 582 (1998)
[7.4] Carbides (MC)
H. Dai, E. W. Wong, Y. Z. Lu, S. Fan, C. M. Lieber, Synthesis and characterization of carbide nanorods, Nature 375, 769 (1995)
[7.5] Nitrogen
Bliznyuk, M. Shen, H. F. Schaefer III, the dodecahedral N20 molecule. Some theorical predictions, Chem. Phys. Let. 198, (3,4), 249 (1992
To contact me: thomaslaudeABC@uminokai.net (Remove ABC, it is against spam.)
The material present here is copyrighted. Key-words: nanotechnology, science, nanotubes.
Birth of the site: August 1999!
Cours de maths-physique-chimie à Rodez |
Cours de flute à Rodez |
Location Honfleur |
Zameho Folk |
Site Scientifique |
Japonais à Rodez |
lire Avec les amis |
Zameho Folk facebook |
Orchestre country Sailor Step |
Thomas Zameho Facebook |
Production Zameho |
Zameho livetonight |
Zameho Amazon |
Spectable |
acteur-fete |
Musique-XYZ |
country-france |
Zameho r-camping |
livetonight Sailor Step